Fruit Battery
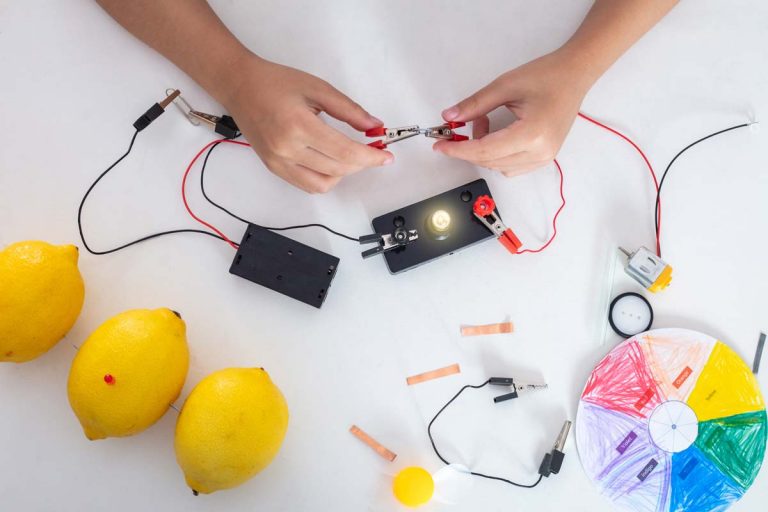 Photo: © iStock kiankhoon
Photo: © iStock kiankhoon - Resource Type
- Experiment
- Subjects
- Chemistry
- Topics
- Energy Scientific Inquiry Sustainability
- Time for activity
- 3 hours
In this activity, students will learn how fruits produce electricity when in contact with metal. They will investigate the relationship between pH and electrical current produced.
- Introduction
-
By 2030, everyone should have access to affordable, reliable and modern energy services. Electrical energy is an important component of our daily life. As we all know, some parts of the world face the lack of electricity and building an electrical generating facility is very expensive. This causes many problems such as the inability to provide schools with electrical power, thus affecting the quality of life. Because of that, many countries are looking into more unconventional and innovative ways to generate electricity.
Did you know that some fruits can produce electricity? This process occurs by converting chemical energy from foods to produce electrical current. One of the common examples of food used are lemons and potatoes.
In this activity, students will learn how fruits produce electricity when in contact with metal. They will investigate the relationship between pH and electrical current produced, and the number of fruits needed to light up a red LED. With this idea, the students will present a solution to provide affordable electricity to rural schools and houses around their area.
- Key Objectives
-
- Identifying the fruits that are the most suitable for power production
- Identifying the relations between the pH of the fruits and electrical conductivity.
- Presenting an idea to generate more affordable electricity to power rural schools / houses by using the idea obtained from the experiment.
- Guiding Questions
-
- Which fruits are the most suitable for power production?
- How many fruits are needed to light up a red LED light?
- What is the relationship between pH and electrical conductivity?
- How can this idea be adapted to supply rural schools and houses with electricity?

/rating_on.png)
/rating_half.png)
/rating_off.png) (25 )
(25 )





